Protein misfolding and aggregation in disease
Mantova, February 12th-14th, 2025
Organizers: F. Chiti, M. Nuvolone, S. Ricagno.

Prof. Stefano Ricagno
We employ several structural biology techniques and many biophysical methods to characterise the structure, dynamics and fold stability of native and aggregated conformations.
Organizers: F. Chiti, M. Nuvolone, S. Ricagno.
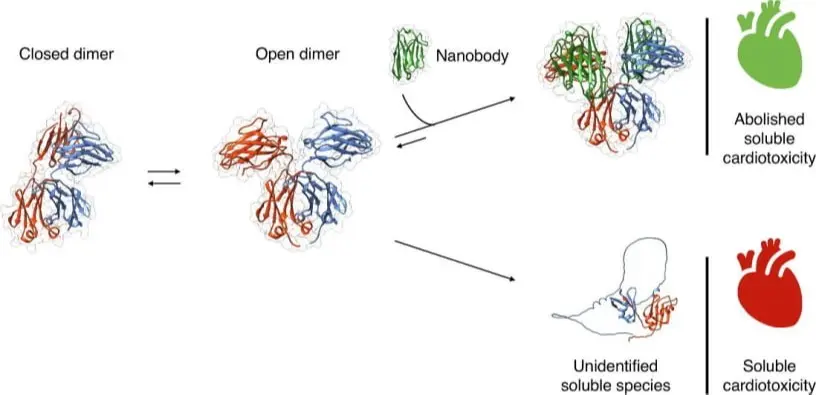
Journal of Molecular Biology
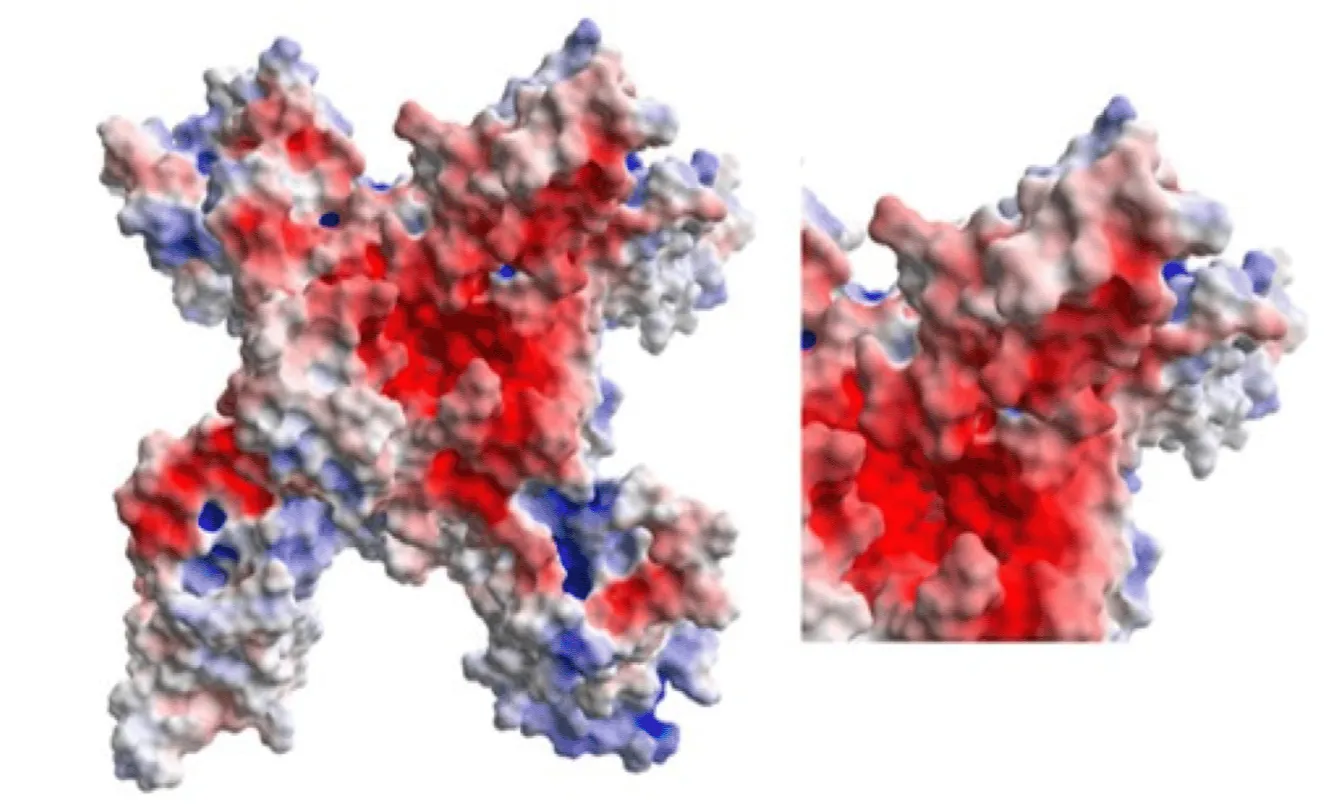
International Journal of Molecular Sciences
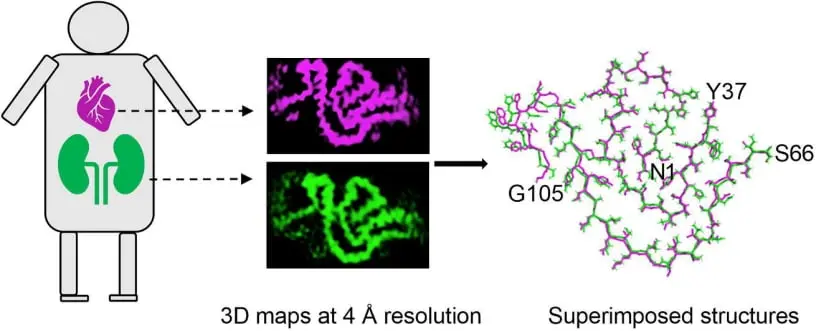
Journal of Molecular Biology
HTML Creator