Project Definition
Background
Exposure to Air Pollution and Cardiovascular Effects
According to the WHO, diseases of the heart and cardiovascular system are the largest single cause of death (accounting for about 2 million deaths) in the European Union. Cardiovascular diseases account for the largest number of premature deaths before 75 years of age. Numerous health studies have shown acute (Schwartz and Marcus 1990; Schwartz and Dockery 1992; Katsouyanni et al. 1997; Katsouyanni et al. 2001; Kinney and Ozkaynak 1991) and chronic (Dockery et al. 1993; Pope et al. 1995; Pope et al. 2004) particulate air pollution exposures to be associated with early death, particularly from cardiovascular disease.(Schwartz and Dockery 1992; Pope et al. 2004) Metals, which are constituents of particulate air pollution, are also associated with CVD.(Yeh and How 1967; Tseng 1977; Chen et al. 1988; Chen et al. 1996; Chen et al. 1995; Chiou et al. 1997; Tseng et al. 2000; Wang et al. 2002; Navas-Acien et al. 2005; Carroll 1966; Voors and Shuman 1977; Voors et al. 1982; Elliott et al. 2000; Navas-Acien et al. 2004; Jeong et al. 2004; Schwartz 1995; Moller and Kristensen 1992; Gerhardsson et al. 1995; Lustberg and Silbergeld 2002; Schafer et al. 2005) Epidemiological and animal studies have suggested many potential mechanisms by which particles may impact health. Airway or parenchymal inflammatory responses to particulate matter (PM) have been hypothesized to be the inciting events of a cascade of pathophysiologic changes in autonomic cardiac, systemic inflammation, and haemostatic activities. All these processes may ultimately lead to the acute cardiac events associated with PM exposure (Lippmann et al. 2003). However, the relative importance of each potential pathways and the steps along these pathways are not well understood, particularly as to how they relate to specific particle components and sources, for which biological pathways are likely to differ. One of the most important gaps in our current knowledge regarding PM-related health effects is the identification of susceptible subjects.(Chen et al. 2007) Recent research findings pointed out obesity as a susceptibility factor to the adverse effects of PM exposure partly due to an increase in particle absorption. A positive correlation between exhaled nitric oxide, a marker of pulmonary inflammation, and Body mass index (BMI) have been shown in healthy adults.(De Winter-de Groot et al. 2005) BMI was associated with a graded increase in the estimated total lung dose of deposited fine particles in an inhalation study of healthy children (6–13 years of age).(Bennett and Zeman 2004) In a panel study of 44 senior citizens, vascular inflammatory response (measured by C-reactive protein) to ambient levels of PM2.5 (particulate matter with aerodynamic diameter ≤2.5μm) averaged over 1–7 days was greater in obese (BMI ≥ 30 kg/m2) than in non-obese subjects.(Dubowsky et al. 2006) A differential autonomic cardiac response (measured as heart rate variability) to metal particulates have been observed between obese and non obese individuals (Chen et al. 2007) Taken together, these findings suggest that obese individuals might represent the best population to investigate PM mediated cardiovascular effects and related pathogenetic mechanisms.
Extracellular vesicles and microRNAs in PM-related cardiovascular effects
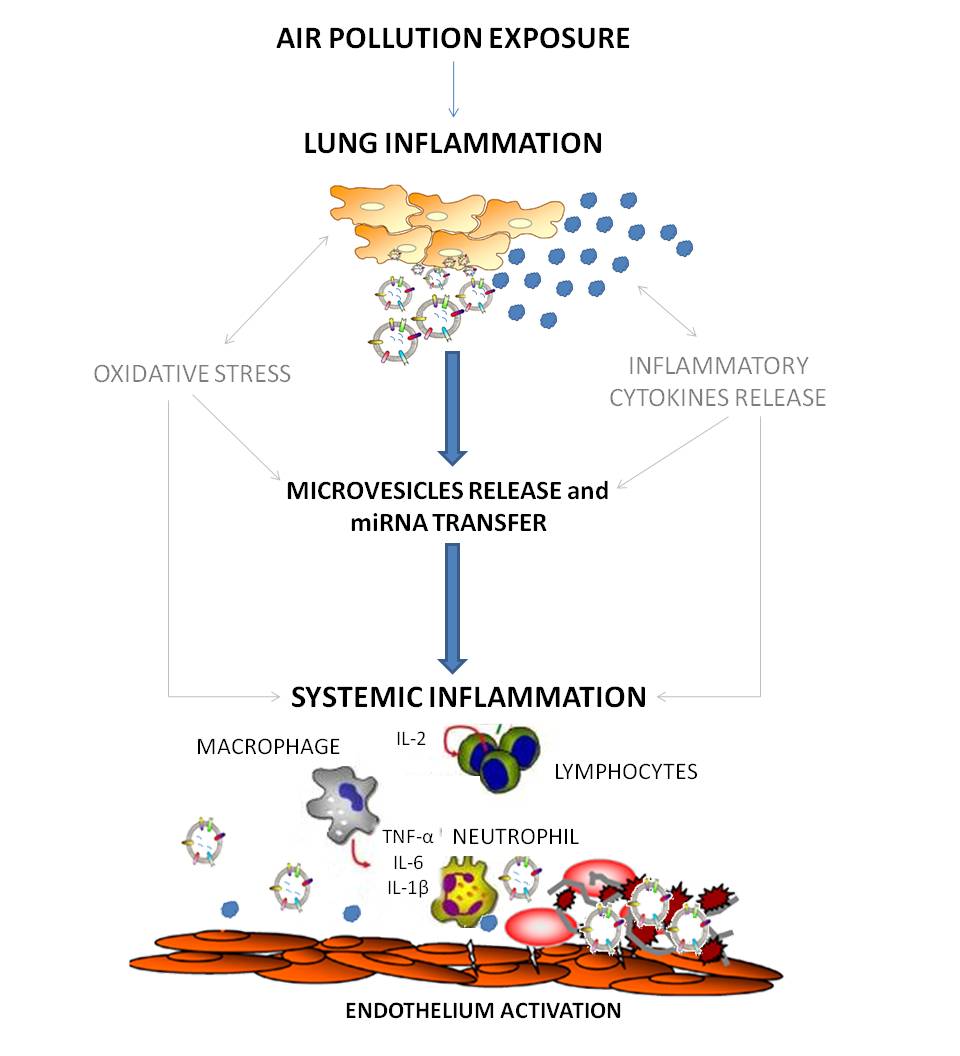
The mechanisms linking PM exposure and cardiovascular disease have not yet been fully elucidated. It has been proposed that inhaled fine particulate matter translocates directly into the systemic circulation through the pulmonary capillary bed, promoting atherothrombosis by breaching endothelial integrity and inciting a local inflammatory reaction.(Nemmar et al. 2001) However, just a very small fraction of these fine and ultrafine particles accumulate in extra pulmonary organs such as the liver and the spleen,(Mills et al. 2006) and currently there is no final evidence that fine particles physically enter and deposit in blood vessels. An alternative hypothesis is that ambient particles produce a strong inflammatory reaction in the lungs followed by the release of proinflammatory mediators that are able to reach the systemic circulation.(Brook et al. 2004; Al-Nedawi et al. 2009) In spite of more than two decades of mechanistic research, the recent statement of air pollution and cardiovascular disease from the American Heart Association remarked that at front of high degree of consistency of the epidemiology findings showing increased cardiovascular risk, the evidence on intermediate mechanisms remains moderate or weak.(Brook et al. 2010) In addition, inflammatory and oxidative responses have little specificity and can be activated by a multitude of trigger, thus limiting our capability to correlate them to air pollution exposure as well as to provide the groundwork for the development of targeted biomarkers and prevention strategies. Beside the release of proinflammatory mediators, cell-derived membrane vesicles are also released, representing another mode of intercellular communication that has recently become the subject of increasing interest.(Al-Nedawi et al. 2009) Extracellular vesicles might be the ideal candidate to mediate the effects of air pollution, since potentially they could be produced by the respiratory system,(Kesimer et al. 2009; Prado et al. 2008) reach the systemic circulation (Orozco and Lewis 2010) and lead to the development of endothelial disfunction (Puddu et al. 2010). Extracellular vesicles are spherical structures limited by a lipid bilayer that can be generated by cells and secreted into the extracellular space. There are various types of secreted membrane microvesicles that have distinct structural and biochemical properties depending on their intracellular site of origin, and these features probably affect their function. Microparticles originating from platelets, endothelial cells and monocytes have been most extensively studied.(Piccin et al. 2007) Platelet microparticles were originally studied because of their procoagulant activity (Piccin et al. 2007) and recent studies have investigated their involvement in the pathophysiology of vascular disorders.(Piccin et al. 2007) They could also participate in a defensive shedding of complement attack complexes(Pilzer et al. 2005) or in deployment of immunomodulating activities.(Valenti et al. 2007) Moreover, extracellular vesicles are released from cell membranes by triggers such as endotoxin encounter, hypoxia or oxidative stress conditions, cytokines release, thrombin production (VanWijk et al. 2003) and could be one of the means used by tissues to adapt to these changes.(Thery et al. 2009) In particular, DNA damaging conditions have been related to activation of the p53 that leads to increased extracellular vesicles secretion.(Lespagnol et al. 2008) Extracellular vesicle membranes are enriched in molecules characteristic of their parent cell and express adhesion molecules on their surface (i.e., ICAM1), which could favor their capture by recipient cells. The fate of extracellular vesicles after binding the surface of recipient cells is not known but recent evidence suggests that they might fuse with recipient cell membranes and deliver their content directly into the cytoplasm of the recipient cells. It has been suggested that extracellular vesicles, after internalization within target cells through surface-expressed ligands, may transfer microRNAs (miRNAs) (Skog et al. 2008; Hunter et al. 2008) enabling intercellular and inter-organ communication in the body.(Hunter et al. 2008) Moreover, miRNA expression in circulating extracellular vesicles has been detected also in plasma of normal subjects and a predictive role of peripheral blood miRNA signatures in human disease has been also hypothesized.(Hunter et al. 2008) MiRNAs are small, endogenous, single stranded noncoding RNAs of 20-22 nucleotides (Andrea Baccarelli and Valentina Bollati 2009) that post-transcriptionally regulate gene expression by either triggering mRNA cleavage or repressing translation.(He and Hannon 2004) One single miRNA can regulate hundreds of mRNAs in interrelated gene pathways and a single mRNA can be targeted by several different miRNAs.(Lewis et al. 2005) Changes in the expression of several miRNAs have been implicated in disease mechanisms that may be related to PM exposure such as oxidative stress (Babar et al. 2008) and regulation of inflammation.(Xiao and Rajewsky 2009) Recently, our group showed in foundry workers of an electric-steel plant facility that air particles, particularly those rich in lead and cadmium, are able to modify miRNAs expression in blood.(Bollati et al. 2010)
Objectives
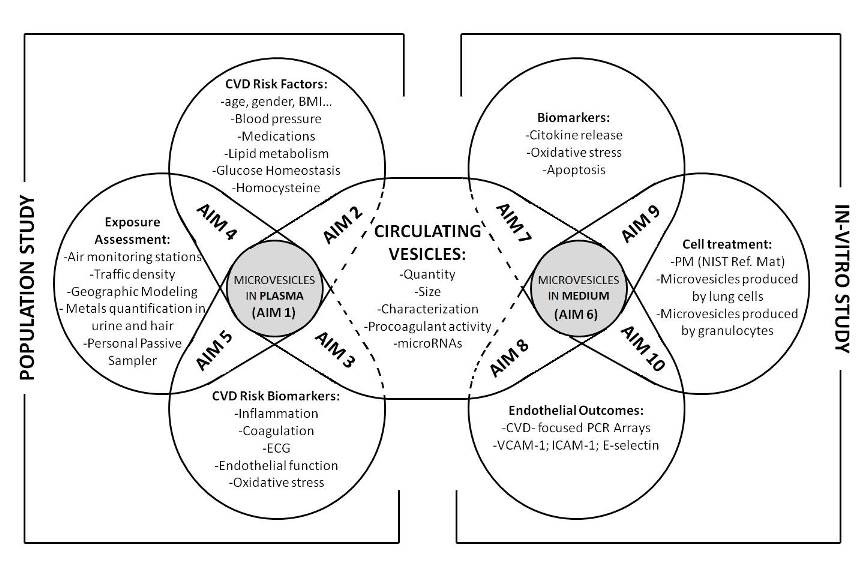
In order to address the effects of environmental air particles and metals on miRNAs contained within plasma exosomes, we have designed the present proposal, based on a two step approach. In this approach a first level of investigation is based on a population study while a second level is based on an in-vitro investigation. The population study (Aims 1-5) will help clarifying what are the modifications in exosomes-associated miRNAs induced by air pollution exposure. Since this part of the study does not allow the investigation of the mechanism at the endothelial level, we will integrate it with the in vitro experiments (Aims 6-10). This second step will help to elucidate the mechanism by which exosomes, eventually produced after air pollution exposure, are able to modify endothelial cells.
Population Study aims: The population study will include 2000 overweighed/obese subjects, at the Center for Obesity and Work (IRCCS Fondazione Ca’Granda – Ospedale Maggiore Policlinico, Milan). This study population has been chosen since obese subjects are supposed to be particularly susceptible to the effects of air pollution.
Specific aim 1. Determine whether exposure to air particles and PM-associated metals can modify exosomes in plasma as: a) quantity b) size c) characterization of the membrane molecules d) procoagulant activity e) miRNAs content
Specific aim 2. Determine whether the changes found in exosomes (Aim 1) are associated with Cardiovascular Disease (CVD) Risk Factors, such as: a) Aging b) Gender c) Smoking Habits d) Alcohol Consumption e) BMI f) Blood Pressure g) Medications h) Lipid Metabolism (Total Cholesterol, HDL-C, Triglyceride, LDL-C, HDL-2, HDL-3, Apolipoprotein A) i)Glucose Homeostasis (fasting and post load glucose, Insulin) j) Homocysteine
Specific aim 3. Determine whether the changes found in exosomes (Aim 1) predict alterations of CVD Risk endpoints, such as: a) Inflammation Markers (Cytokines) b) Coagulation Markers (Platelet Count and coagulation factor VIII, Prothrombin Time [PT] and Partial Thromboplastin Time [aPTT]) c) ECG (QRS, PR waves and heart rate) d) Endothelial function (ICAM-1, VCAM-1, C-reactive protein) e) Oxidative stress (8-OHdG and isoprostane [8-isoPGF2α])
Specific aim 4. Determine whether exposure to air particles and PM-associated metals are associated with selected CVD Risk Factors, such as: a) BMI b) Blood Pressure c) Lipid Metabolism (Total Cholesterol, HDL-C, Triglyceride, LDL-C, HDL-2, HDL-3, Apolipoprotein A) d) Glucose Homeostasis (fasting and post load glucose, Insulin) e) Homocysteine
Specific aim 5. Determine whether exposure to air particles and PM-associated metals predict alterations of CVD Risk Biomarkers (listed in Specific Aim 3).
In vitro study aims: We will integrate the human study with an in-vitro model. We will use a system made of paired cell cultures (A549 lung cells and HUV-EC-C endothelial cells or granulocytes and HUV-EC-C endothelial cells) to clarify if exosomes produced by lung/granulocytes can directly interact with vascular endothelial cells
Specific aim 6. Determine in vitro whether exposure of lung cells/granulocytes to PM and PM-associated metals can modify exosomes release in cell medium as: a) quantity b) procoagulant activity c) miRNAs content
Specific aim 7. Determine whether the changes found in exosomes (produced by lung and granulocytes exposed to PM) predict alterations of CVD Risk Biomarkers, such as: a) Pro-Inflammation Markers (Cytokine release) b) Oxidative stress (8-OHdG and isoprostane 8-isoPGF2α) c) Cell Mortality
Specific aim 8. Determine whether the changes found in exosomes (producted by lung and granulocytes exposed to PM) are associated with changes in endothelial cells, such as : a) Pro-Inflammation Markers (Cytokine release) b) Oxidative stress (8-OHdG and isoprostane 8-isoPGF2α) c) Mortality
Specific aim 9. Determine whether the changes found in exosomes predict alterations of CVD Risk Biomarkers, such as: a) Pro-Inflammation Markers (Cytokine release) b) Oxidative stress (8-OHdG and isoprostane 8-isoPGF2α) c) Mortality
Specific aim 10. Determine whether endothelial cells treated with purified exosomes (producted by lung and granulocytes exposed to PM) show: a) Alterations in RNA expression evaluated by a CVD- focused PCR Arrays. b) Alterations in VCAM-1, ICAM-1 and E-selectin proteins.